Anonymization and Redaction Automation
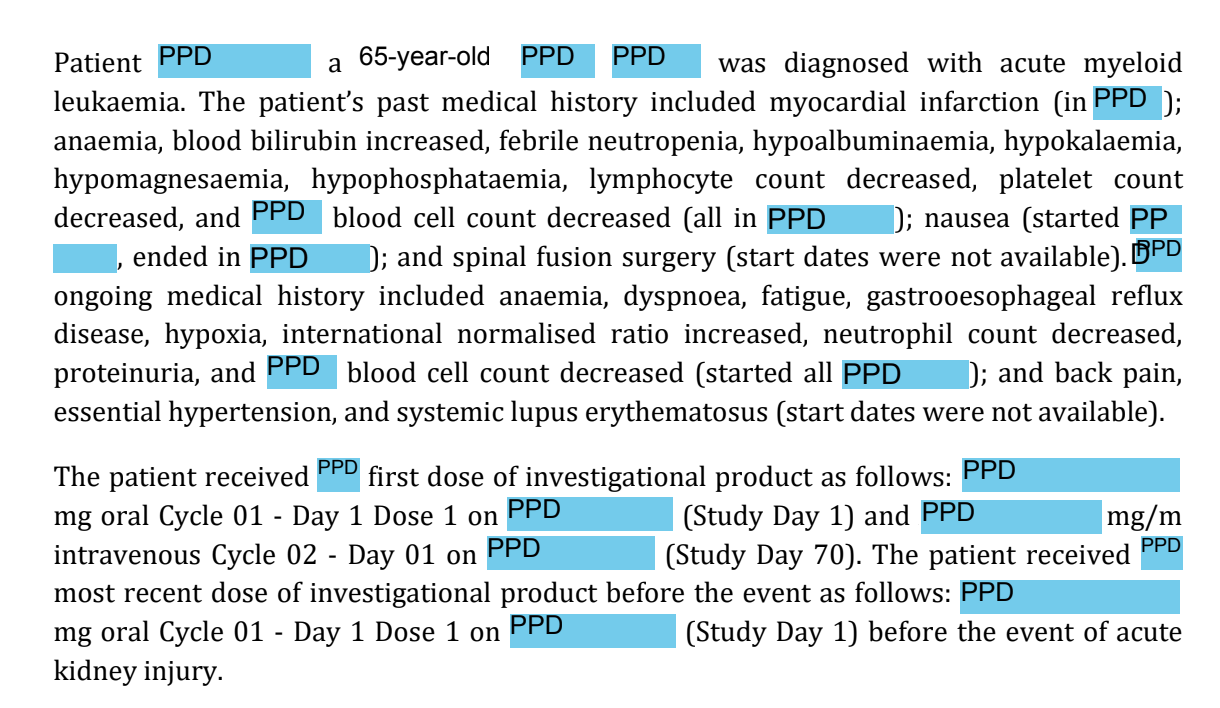
Anonymization and redaction are critical steps in protecting the identity of clinical trial participants and safeguarding proprietary business information. Redaction, a subset of anonymization, involves masking personally identifiable information (PII), protected personal data (PPD), and commercial confidential information (CCI) in compliance with global regulatory standards. As clinical datasets grow in complexity and size, manually redacting sensitive content becomes increasingly time-consuming and prone to error.
Redaction Scout automates this process using generative AI and the Narrativa Knowledge Graph, enabling accurate, efficient, and compliant redaction across large-scale clinical documents.
- Automatically scans and redacts sensitive information from clinical documents, regardless of length or complexity
- Supports PII, PPD, and CCI redaction in accordance with FDA, EMA, Health Canada, and other global guidelines
- Fully customizable Anonymization Schema Configuration for flexible, rule-based data masking
- Integrates seamlessly with SDTM and ADaM datasets for comprehensive data protection
- Employs blacklist and pattern-matching capabilities to detect and redact high-risk terms and identifiers
- Ensures compliance with data privacy regulations while supporting transparency in public disclosures
Benefits
Saves you time by checking thousands of pages of clinical documents with a few clicks
- Maintains consistency and accuracy across entire documents
- Uses customizable parameters to improve the anonymization and redaction processes
- Improves efficiency by proactively approaching data and document anonymization
- Boosts your team’s efficiency by sparing them from going through every page manually
Learn more: Redaction and Anonymization with Generative AI
Anonymization is the process of removing information that could be used to identify study participants in both direct and indirect manners, like gender, age, and country, among others. Redaction is a subclass of anonymization that involves data masking to protect clinical trial participants. This can be personally identifiable information (PII), personal protected data (PPD), or sensitive and/or proprietary business information, known as commercial confidential information (CCI). It is important to note that regardless of the masking technique, health authorities, such as the Food and Drug Administration (FDA), Health Canada, and the European Medicines Agency (EMA), have their own rules and guidelines when it comes to permitted levels of anonymization and redaction. Depending on the context and the health authority, anonymization could be done while the study is being conducted or after the documents have been submitted to the governing bodies.
Clinical trial participants’ data are often collected in large databases, like the Study Data Tabulation Model (SDTM) and the Analysis Data Model (ADaM), that contain hundreds or thousands of entries pertaining to the study subjects. The information contained in these datasets is used to develop clinical documents, which could be thousands of pages long or even longer in larger trials. Therefore, these datasets should ensure that participants’ information is masked to avoid identifying the person. So, how can Narrativa’s Generative AI technologies help with this?
The use of Narrativa’s redaction solution will help you anonymize and redact sensitive information with the press of a few clicks. Our solution will scan through the clinical documents, regardless of their size, to determine the information eligible for masking.
With our anonymization and redaction solution, the information of your study population and your organization’s commercial secrets are kept without compromising the policies of health authorities and transparency laws.