Clinical Study Reports Automation
Benefits
- Enhanced Efficiency: Streamlines processes and reduces authoring time, leading to greater operational efficiencies.
- Accelerated Approvals: Speeds up the regulatory submission and approval process.
- Cost Reduction: Significantly lowers costs and overhead associated with CSR creation.
- Fewer Reviews: Reduces the need for multiple review cycles, minimizing effort and errors.
- Improved Compliance: Ensures better regulatory adherence and alignment with industry guidelines.
Underlying Technology
- Knowledge Graphs: Organizing and linking complex data for deeper insights.
- Generative AI: Crafting coherent and compliant narratives.
- Entity Extraction: Identifying and extracting key medical and regulatory entities.
- Data Clustering: Grouping relevant data points for structured reporting.
- Natural Language Processing (NLP): Transforming clinical data into readable and meaningful text.
Narrativa’s answer to regulatory affairs automation
Narrativa’s CSR Atlas leverages generative AI to transform clinical trial data into comprehensive Clinical Study Reports (CSRs). Its advanced capabilities extend beyond text generation to include information extraction, question answering, and contextual data interpretation—reshaping the way clinical trials are designed, documented, and reported.
Automation of clinical report writing
CSR Atlas enables pharmaceutical companies to automate the creation of Clinical Study Reports (CSRs), streamlining workflows, reducing costs, and ensuring regulatory compliance. By minimizing human error and cutting down on review cycles, this AI-powered tool enhances overall efficiency. CSR Atlas supports key use cases such as the integration of Tables, Listings, and Figures (TLFs) and patient safety narratives, significantly reducing the manual workload for medical writing teams.
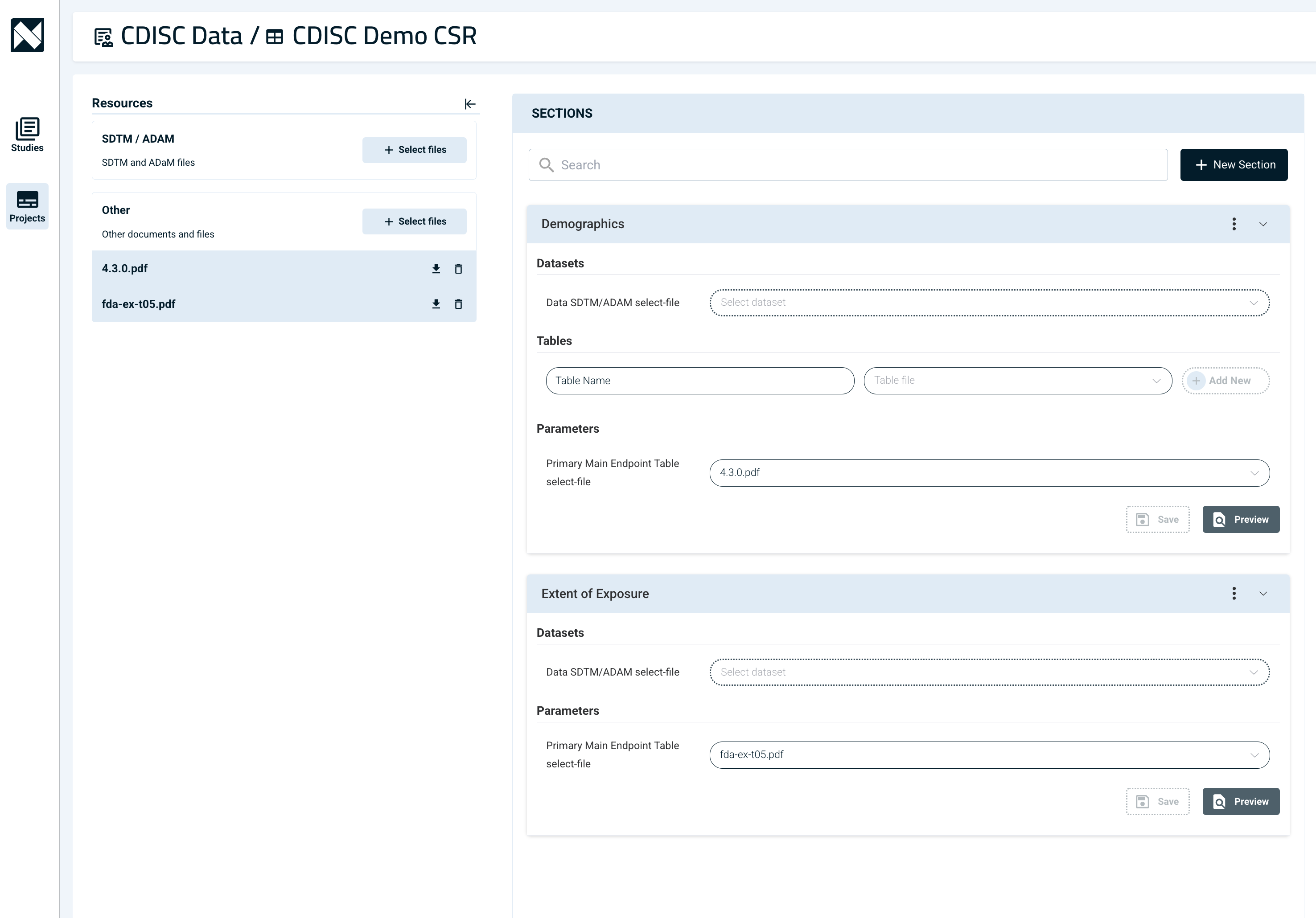
How does CSR Atlas work?
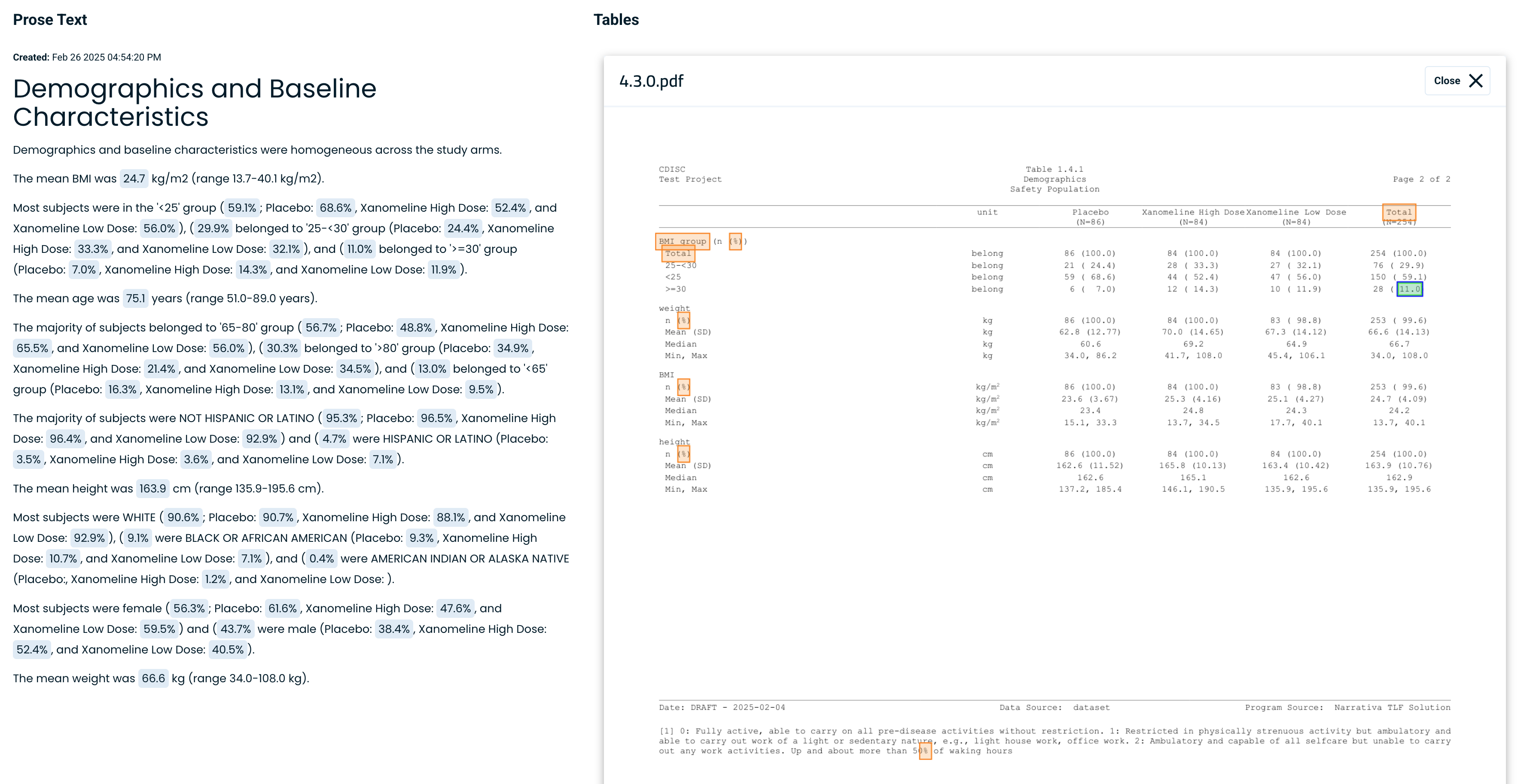
CSR Atlas automates the writing of Clinical Study Reports by extracting data directly from Tables, Listings, and Figures (TLFs) and ADaM datasets, converting them into clear, structured narrative text. Instead of manually reviewing complex tables, the AI processes TLFs from multiple formats, maps the data into the Narrativa Knowledge Graph, and generates accurate, submission-ready prose—saving time and reducing errors.
CSR Atlas supports both system-generated and user-defined prompts, allowing for flexible, high-quality content tailored to specific reporting needs.
Traceability and validation
Since CSR Atlas generates the initial draft, medical writers are responsible for reviewing and validating the content for accuracy.
To support this, the platform allows users to click on any data point in the narrative and instantly trace it back to its source in the original table or dataset. This feature simplifies the quality assurance process, enabling fast, precise verification and edits while keeping full editorial control with the medical writer.
Narrativa Knowledge Graph®
The Narrativa Knowledge Graph® integrates various data management methodologies, including:
- Interlinked Databases: Providing structured entity descriptions.
- Semantic Knowledge Bases: Enabling data interpretation and inference.
By combining deep learning with knowledge graphs, Narrativa can process vast datasets, extract key insights, and generate contextualized reports.
Advanced Statistical and Analytical Integration
Narrativa’s CSR Automation solution incorporates:
- Statistical testing for hypothesis validation
- Confidence interval calculations
- Risk assessments (relative risk, risk ratios)
- Frequentist and data augmentation techniques
More features: variability and rich language
Narrativa’s CSR automation solution is designed to generate high-quality content with ease. By training on a provided corpus, the system adapts to each organization’s preferred writing style, producing narratives with rich language and variability when needed.
Using deep learning, the platform extracts key topics, synonyms, and sentence structures from large volumes of clinical trial data to mimic the desired tone and format. Users retain full control over the output, with the ability to refine text as needed through an iterative process.
This approach reflects Narrativa’s mission to apply AI responsibly—turning complex clinical data into clear, compliant documentation that serves both science and society.
Learn more: CSR Generative AI Automation
The pharmaceutical industry is undergoing a data revolution, with AI playing a pivotal role in transforming raw data into actionable insights. Traditionally, empirical analysis has been the foundation for identifying patterns, testing hypotheses, and evaluating treatment efficacy. Now, AI is set to amplify this process, enabling faster knowledge dissemination and regulatory compliance.
Overcoming regulatory compliance challenges
Regulatory compliance is a critical component of clinical trials, yet the manual creation of CSRs remains a resource-intensive task. Automating these reports allows pharmaceutical companies to:
- Optimize operational efficiency
- Reduce costs and manual workload
- Reallocate resources to higher-value research and development activities
By embracing AI-driven CSR automation, the pharmaceutical industry can expedite drug development, improve regulatory submissions, and ultimately enhance patient outcomes.