Patient Narratives Automation
Simplify research by automating patient reports
Narrativa’s Generative AI Automation Platform uses deep learning language algorithms to automate the generation of patient safety narratives
Solution trusted by

Up to a 6 week
Reduction in authoring time
50%
Reduction in regulatory reviews
30 seconds
To generate the first draft
PATIENT SAFETY NARRATIVES AUTOMATION
Patient safety narratives are a key part of clinical study reporting. The automation of patient safety reports can help save time by automating the repetitive elements.
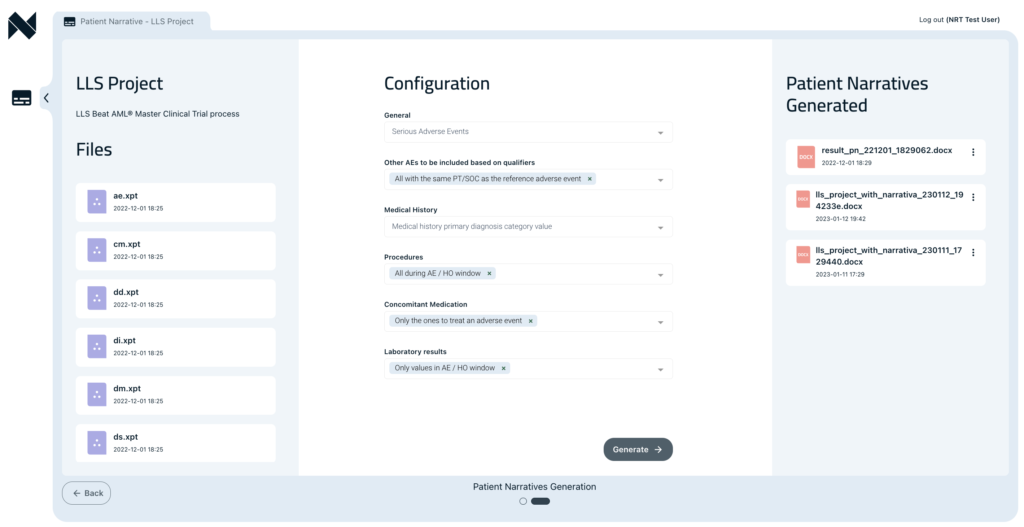
Benefits
- Faster authoring times for narratives’ first draft
- Generation of pre-formatted and editable narratives with the flexibility for modification
- Improved consistencies in narrative generation
- Improvement of the revision process
- Significant reduction in reporting conflicts and inconsistencies
- Improved quality control (QC) process through audit trail and version tracking
- Regulatory compliance
- Acceleration of the final approval process
- Delivery of patient safety narratives in batches

Patient Narratives AI Automation
Regulatory Compliance in the Pharmaceutical Industry
Patient safety narratives are brief summaries of adverse events experienced by patients during clinical trials. Patient safety narratives establish any causal relationship between the adverse events experienced by the patient and the drug being investigated.
Just like Tables, Lists, and Figures (TLFs), safety narratives are a key part of clinical study reporting through all phases of clinical trials (Phases I-IV) and are an integral part of the safety review process that need to be submitted as a part of the Marketing Authorization Application (MAA) dossier.
The patient safety narrative describes the following:
- Nature of the event, its intensity, and outcome
- Clinical course leading to the event
- An indication of timing relevant to the administration of the drug being investigated
- Relevant laboratory measures
- Actions taken with the drug being investigated in relation to the event
- Treatments or interventions undertaken
- Post-mortem findings, if any
- Investigator and sponsor opinion on causality
Traditionally, the task of reporting adverse events during clinical trials has been conducted manually after going through the case report forms (CRFs) and entering each patient’s data manually. The task of generating safety narratives is complex, which can be compounded during large clinical trials for chronic conditions. As safety narratives are pivotal to clinical study reporting obligations, automated procedures for reporting patient safety narratives can significantly reduce time and cost burdens.
Automated generation of Patient Safety Reports
The automated generation of patient safety reports can achieve significant reductions in time and cost while enforcing the use of a common data source for the contents of this deliverable. This can be achieved by leveraging Generative AI to apply standard reasoning for individual investigational drugs so as to automate the repetitive sections of CSR reporting. This automation process is especially helpful for large clinical trials involving thousands of patients, and when time is a constraint in the preparation and submission of patient narratives to the proper regulatory authorities. The process of automating safety narratives can help raise the overall quality of the data for regulatory submissions and simultaneously reduce the time required for the generation of narratives.
Narrativa automates patient safety narratives using Generative AI in combination with Narrativa Knowledge Graph® directly from different data sources:
- Case Report Forms (CRFs)
- Council for International Organizations of Medical Sciences (CIOMS) forms
- MedWatch forms
- Data Clarification Forms (DCFs)
- Clinical database listings
Contact us to learn more about how Narrativa’s Generative AI Automation Platform can transform your business through Patient Safety Narratives Automation.
or Contact us



