Narrativa joins forces with InteliNotion to help the Pharmaceutical industry
Narrativa joins forces with InteliNotion to help the Pharmaceutical industry



New Partner
New Partner
New Partner
By Sofía Sánchez González
We are delighted to announce that Narrativa has joined InteliNotion to help pharmaceutical companies in the treatment and management of their data. This news comes on the heels of an equally exciting development, the closure of our 1.3 million euro financing round to accelerate the drug approval process with Natural Language Generation (NLG). Through the agreement with InteliNotion however, Narrativa becomes an even more powerful ally to the Life Sciences industry, able to serve both pharmaceutical companies and clinical research organizations (CROs) better, as our text automation services will be integrated into the InteliNotion platform.
About InteliNotion
InteliNotion is a US-based organization that helps businesses manage their documentation via its advanced SaaS platform. Among the services offered by InteliNotion are:
- Document generation: InteliNotion creates new documentation by repurposing content from one or more existing documents.
- Anonymization and redaction: Anonymization and redaction are necessary to comply with the requirements of EMEA Policy 70 when handling personally identifiable information from clinical data sets.
- Information model management: InteliNotion defines structures, policies and associated metadata for any type of information managed on the platform.
- Systems integration: Integration with external systems and repositories are implemented using an integration framework.
To learn more about InteliNotion, please visit its website.
The pharmaceutical sector needs NLG
Pharmaceutical companies spend over $1.5 billion USD on regulatory documentation per year and the average time to complete the necessary documentation and obtain FDA approval of a new drug might take up to a full year. The extensive time spent on regulatory documentation significantly increases the time-to-market for new drugs.
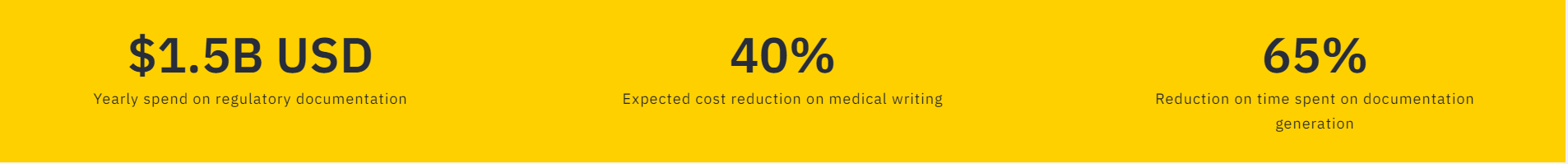
Pharmaceutical companies spend over $1.5 billion USD on regulatory documentation per year
Our Artificial Intelligence (AI) writing assistant uses advanced NLG to run through millions of data points from clinical trials generating readable and comprehensible written medical narratives that increase accuracy and drastically reduce the time and cost to create critical (and laborious) regulatory documents.
Our solutions
Our solutions for Life Sciences and Healthcare include:
- Clinical Study Reports: Medical writers spend huge amounts of time writing clinical study reports (CSRs), time which could be better spent on higher-value tasks. Narrativa uses NLG with advanced AI to transform clinical trial data into clinical study reports.
- Patient Safety Narratives: Patient safety narratives are a key part of clinical study reporting. The automation of these reports helps save time by eliminating the need for repetitive editing.
- eCTD Dossier: Automating the eCTD filing process helps Pharma companies to improve efficiencies and substantially reduce costs and time to market.
- Tables, Lists, and Figures (TLFs): They summarize data sets into easily readable formats that medical writers can use to write the CSR. The conventional way of creating them is tiresome and requires significant resources. Explore how Narrativa automates this process.

Automation of Clinical Trials Reports
Learn more about our services for Life Sciences and Healthcare here.
About Narrativa
Founded in 2015, Narrativa was created from the belief that technology can be used to transform the world in a positive way. Recognized by Samsung as one of the most important companies in the NLG field, Narrativa supports businesses that want to grow by anticipating trends and transforming data into human language in real time. Through its artificial intelligence system and experience in machine learning, Narrativa produces automated content for diverse industries, including finance, pharmaceuticals, insurance, media, and gaming among others.
About Narrativa
Narrativa® is the global leader in generative AI content automation. Through the no-code Narrativa® Navigator platform and the collaborative writing assistant, Narrativa® Sidekick, organizations large and small are empowered to accelerate content creation at scale with greater speed, accuracy, and efficiency.
For companies in the life sciences industry, Narrativa® Navigator provides secure and specialized AI-powered automation features. It includes complementary user-friendly tools such as Clinical Atlas, Narrative Pathway, R-Developer for TLFs, and Redaction Scout, which operate cohesively to transform clinical data into submission-ready regulatory documents. From database to delivery, pharmaceutical sponsors, biotech firms, and contract research organizations (CROs) rely on Narrativa® to streamline workflows, decrease costs, and reduce time-to-market across the clinical lifecycle and, more broadly, throughout their entire businesses.
The dynamic Narrativa® Navigator platform also supports non-clinical industries such as finance, marketing, and media. It helps teams drive measurable impact by creating high-quality, scalable content on any topic. Available as a self-serve SaaS solution or a fully managed service, built-in AI agents enable the production, refinement, and iteration of large volumes of SEO-optimized news articles, engaging blog posts, insightful thought leadership pieces, in-depth financial reports, dynamic social media posts, compelling white papers, and much more.
Explore www.narrativa.com and follow on LinkedIn, Facebook, Instagram, and X. Accelerate the potential with Narrativa®.

